Complete Surgical Instrument Traceability with Vision AI, OCR, and RFID Tags
- Vathslya Yedidi
- June 16, 2025
Surgical instruments play a central role in the operating room, but tracking their use, sterilization, and location across procedures is still a persistent challenge. Traceability, the ability to monitor each instrument’s journey from use to reprocessing and back, is essential for operational efficiency, patient safety, and regulatory compliance.
With global surgical volumes surpassing 310 million procedures annually and widespread hospital-acquired infection risks, effective traceability is also more critical. Traditional methods such as manual logs or barcode stickers lead to delays, errors, and compliance issues.
Surgical instrument traceability with Vision AI, OCR, and RFID Tags enables real-time, closed-loop tracking across the entire instrument lifecycle. Combining automation with data capture and visual verification, these technologies help healthcare facilities improve accuracy, streamline workflows, and reduce risk.
This blog explores how tracking and traceability of surgical instruments with Vision AI, OCR, and RFID Tags work together to adopt safer surgeries, improved operations, and better workflows in healthcare systems.
Instrument Handling Process from Surgery to Sterilization
Surgical instruments move through a standardized sequence of steps after every procedure to ensure they are clean, functional, and sterile before reusing. This process is essential for infection control, patient safety, and operational efficiency. The lifecycle typically consists of seven stages:
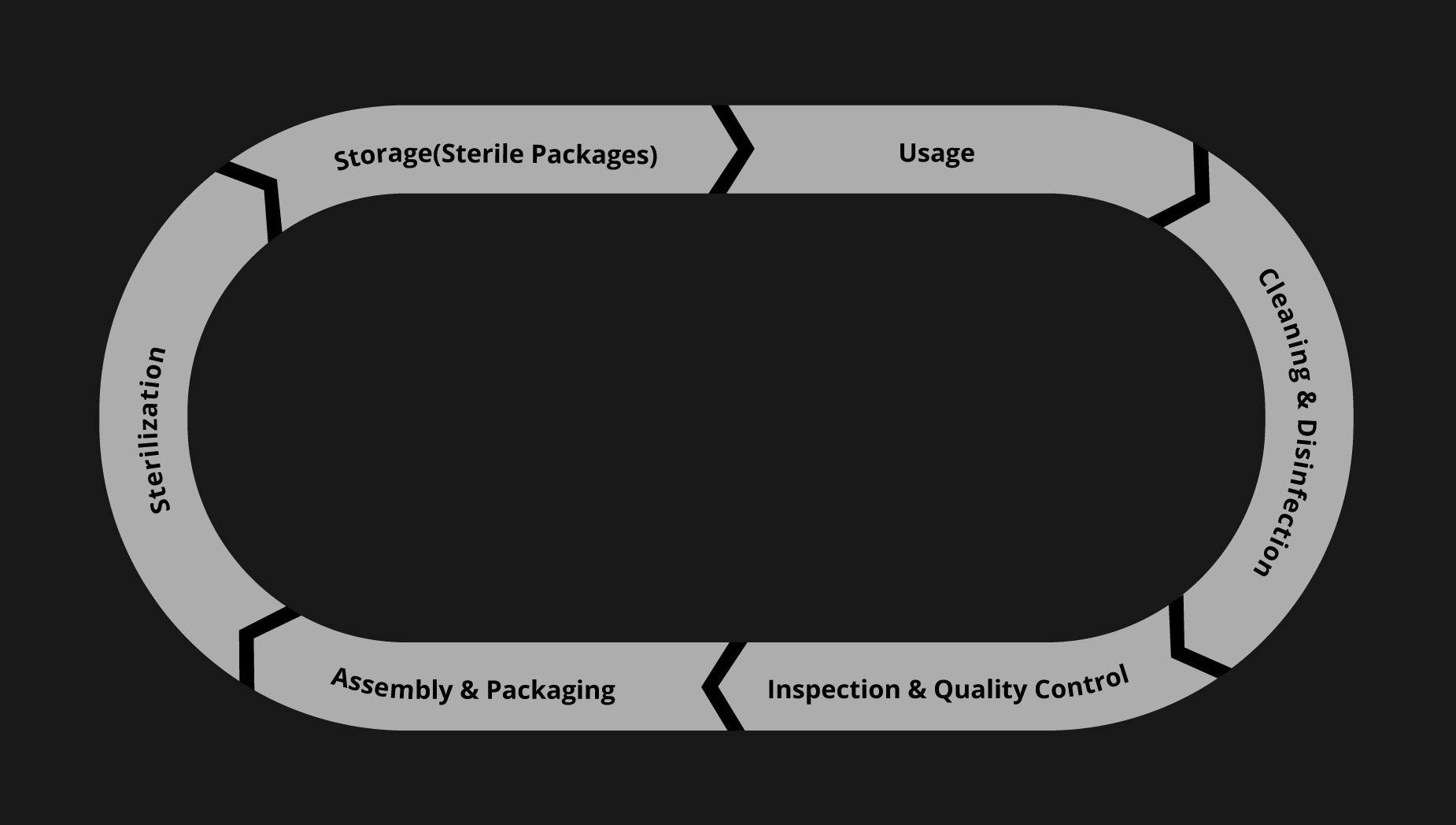
- Instrument Use: Instruments are selected and used during surgical or clinical procedures. Once used, they are classified as contaminated and sent for reprocessing.
- Cleaning and Disinfection: Instruments are cleaned to remove visible contaminants such as blood, tissue, and debris. This process may include manual scrubbing or mechanical washing, followed by disinfection to reduce microbial presence.
- Inspection and Quality Control: After cleaning, instruments are visually and functionally inspected for damage, wear, or defects. Any instrument that does not meet quality standards is either repaired or removed from circulation.
- Assembly and Packaging: Instruments are grouped into trays based on specific procedures. Each tray is assembled using a standardized checklist and then packaged in wraps or rigid containers to prepare for sterilization.
- Sterilization: To ensure complete microbial elimination, the packaged trays undergo sterilization, typically through steam autoclaving or low-temperature processes.
- Storage: Sterile trays are stored in a controlled environment to maintain sterility until needed. Labeling, shelf-life tracking, and environmental monitoring help preserve compliance and readiness.
- Redistribution for Reuse: When a procedure is scheduled, the right tray is retrieved from storage and delivered to the operating room. Once the instruments are used, the cycle begins again.
Why Traceability Is Essential in Tracking Surgical Instruments
Manual or semi-automated tracking systems fall short of meeting the complex demands of modern surgical environments. These limitations manifest in several key areas impacting operational efficiency, patient safety, and regulatory compliance.
1. Lack of Centralized Visibility
Traditional systems do not provide ongoing visibility once surgical kits are dispatched to various departments or facilities. This disconnection leads to delays in kit turnaround, inefficient replenishment cycles, and uncoordinated inventory management across sites, ultimately resulting in increased costs and surgical delays.
2. Incomplete or Missing Instrument Trays
Manual tracking may detect that a tray is incorrect, but it typically cannot identify exactly which instruments are missing. This creates bottlenecks in the reprocessing cycle, delays surgical preparation, and introduces a higher risk of human error during instrument assembly and verification when tools appear visually similar.
3. Disrupted Billing and Inventory Tracking
Without automated confirmation of instrument use and return, billing processes are delayed, and usage data becomes fragmented. This affects financial reconciliation and undermines the ability to track instrument readiness, leading to shortages or overstocking that disrupts surgical planning.
4. Errors Due to Tool Similarity
Many surgical instruments are visually alike, making them difficult to distinguish during manual counts. This can lead to tray mismanagement, miscounts, and inefficient restocking under the pressure of high surgical volumes and tight turnaround times.
5. Audit and Compliance Risks
Manual logs do not meet the traceability standards required by healthcare regulators. Inadequate documentation of sterilization cycles, maintenance records, and instrument usage exposes hospitals and vendors to audit failures, financial penalties, and potential contract losses.
Layered Traceability of Surgical Instruments Tracking with Vision AI, OCR, and RFID Tags
Manual tracking systems create gaps where instruments disappear between departments, get miscounted during busy shifts, or arrive incomplete at the OR. These issues cause surgical delays, increase operational costs, and create audit vulnerabilities for healthcare facilities. Advanced traceability systems operate through a multi-technology architecture. This layered approach enables real-time visibility and validation across all stages of an instrument’s use and reprocessing.
1. Item-Level Identification and Real-Time Tracking with RFID Tags
- Unique ID for Every Tool: Each surgical instrument is tagged with an RFID chip, providing a persistent, scannable identifier.
- Tray-Level Scan Automation: Complete trays can be scanned in seconds, ensuring all instruments are accounted for pre- and post-procedure.
- Return and Sterilization Logs with Time Stamps: RFID readers automatically log each movement, from sterilization to return.
- Audit-Readiness and Inventory Optimization: Detailed logs support regulatory audits and streamline inventory forecasting and replenishment.
2. Visual Verification at Every Stage with Vision AI
- Overhead Camera Scans for Tray Validation: Vision AI-enabled cameras analyze tray layouts during packing for accuracy and completeness.
- Detect Missing, Misplaced, or Damaged Instruments: Vision AI models flag inconsistencies, such as missing tools, incorrect positioning, or visible damage.
- Intraoperative Monitoring for Real-Time Usage Detection: Vision AI systems monitor the usage of surgical instruments and ensure all tools are recovered before wound closure.
3. Bridging Physical and Digital Records with OCR
- Extracts Serial Numbers, Barcodes, and Handwritten Labels: OCR digitizes etched codes, barcode stickers, and handwritten notes.
- Digitizes Count Sheets and Links to Instrument Records: Physical records are converted into structured, searchable data linked to the instrument’s digital trail.
- Improves Compliance, Auditability, and Traceability: Digital documentation enables rapid access for audits, investigations, and sterilization reviews.
Inside the Dashboards of Surgical Instrument Traceability with Vision AI, OCR, and RFID
1. Vision AI Dashboard
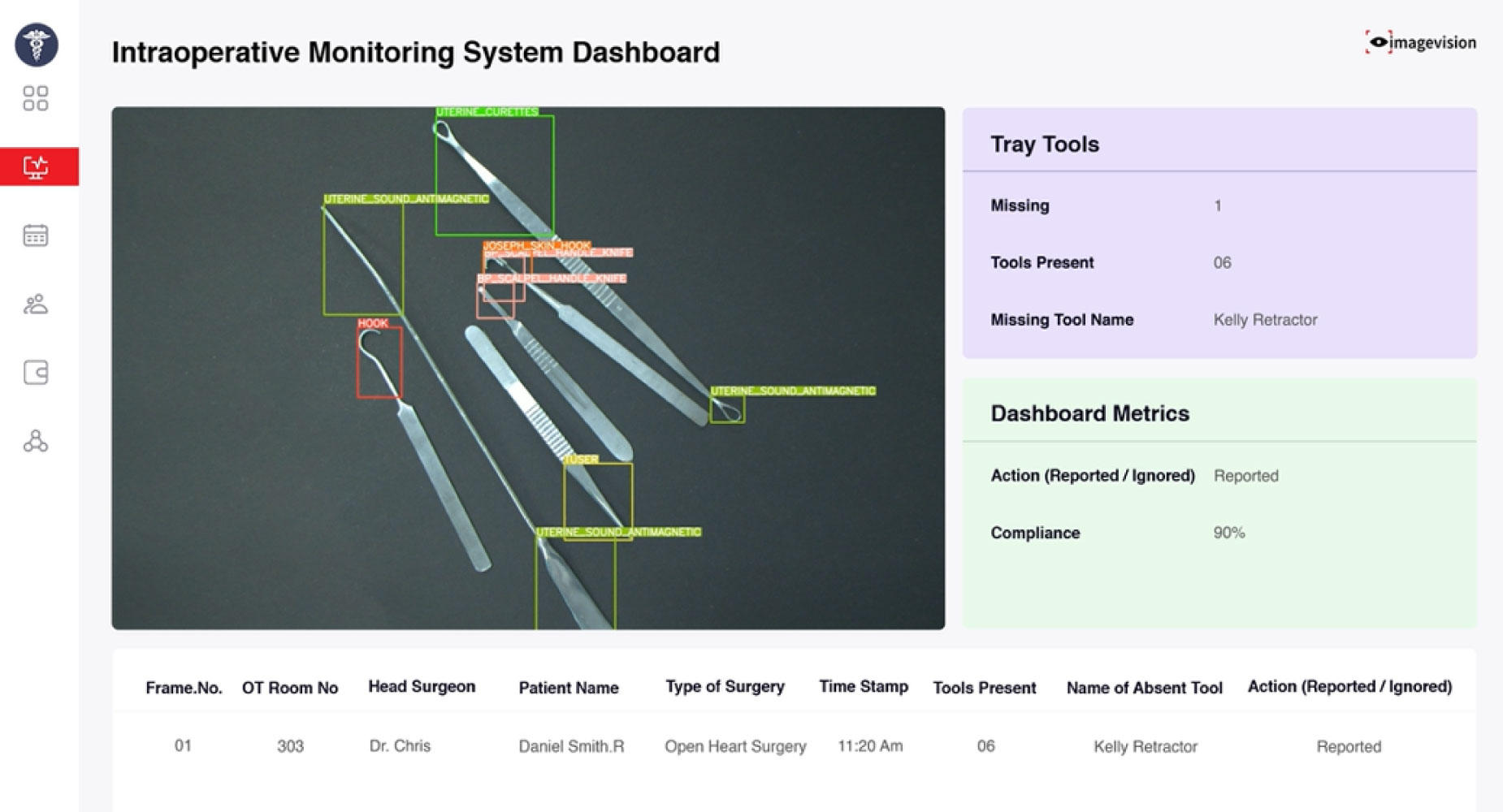
The dashboard displays real-time tray scans using overhead cameras. It auto-detects and counts instruments, verifies their position, and compares the layout to standard tray configurations. Any missing, extra, or misplaced tools are flagged instantly. The dashboard supports pre-op and post-op checks, helping ensure tray accuracy and surgical safety.
2. OCR Dashboard
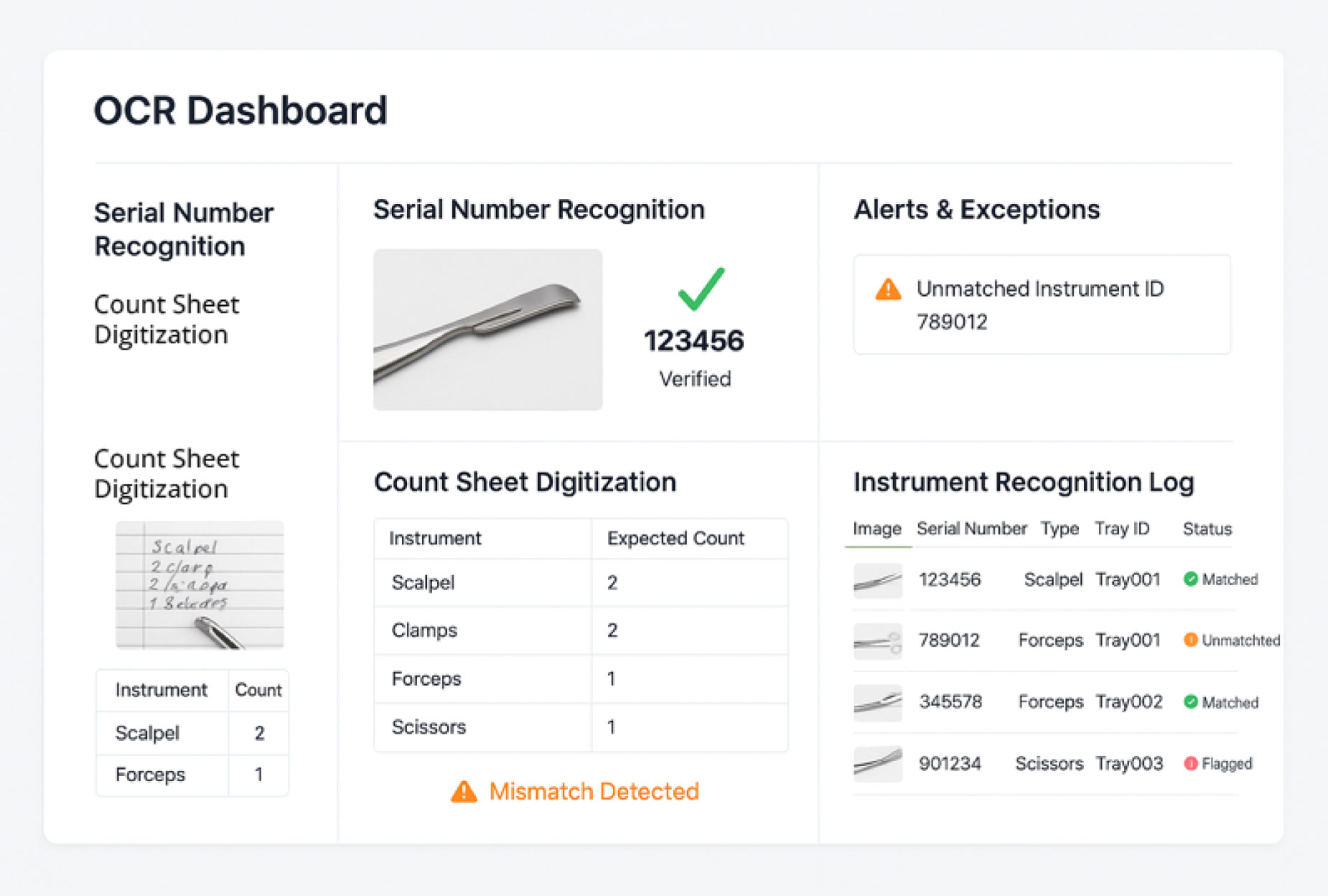
The OCR dashboard captures data from etched serial numbers, barcodes, and instrument labels. It also digitizes printed or handwritten count sheets. All extracted information is matched to tray records and displayed for quick review. Discrepancies are flagged, supporting accurate instrument tracking and digital recordkeeping.
3. RFID Dashboard
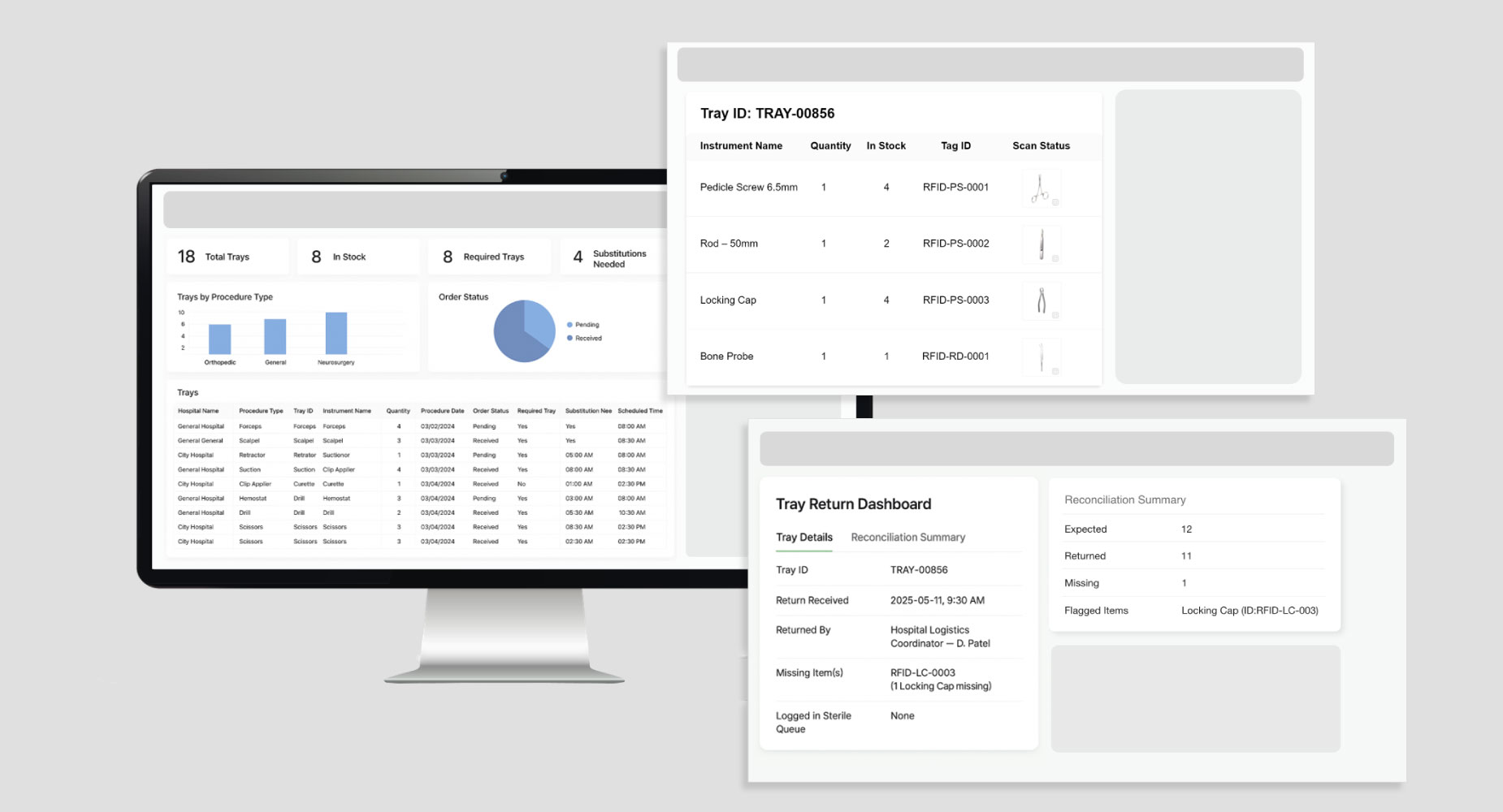
Logs RFID scan data across key checkpoints like packing, sterilization, and return. It shows real-time tray status, highlights missing items, and records each scan with a timestamp. The dashboard ensures accurate reconciliation and tool-level tracking, supporting audits, inventory, and billing reporting.
Conclusion
Surgical instruments pass through multiple critical stages between use and reuse, each requiring precision, documentation, and coordination. Gaps in tracking can lead to delayed procedures, incomplete trays, compliance risks, and safety concerns that affect staff and patients.
Combining RFID, Vision AI, and OCR into a unified traceability system, healthcare facilities can set up end-to-end visibility and control over every instrument in circulation. Each tool’s identity, usage, condition, and sterilization status are captured and validated at every step, enabling faster workflows, fewer errors, and improved accountability.
As surgical environments grow more complex, adopting technologies that support accurate, real-time traceability helps maintain operational reliability and meet rising care quality and safety standards.
To learn more about how this traceability approach can fit into your surgical workflows, get in touch